Managing Your Incontinence
Continence Products find an essential role in enhancing the quality of life of those that live with incontinence who are:
-
- unable to be cured
- are awaiting treatment
- treatment is unavailable
- are awaiting treatment to take effect
- who elect not to pursue cure options (Rottenden, 2010)

Good quality product selection can radically affect the quality of life of people who live with incontinence. Those who are difficult to treat often have the most severe incontinence, therefore are the most dependent on quality products that will help to achieve social continence (Rottenden, 2010).
Those who live with moderate to severe urinary incontinence are traditionally reliant on disposable continence aids.
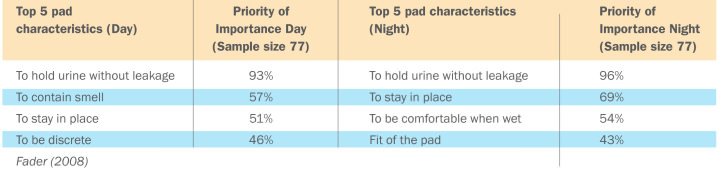
Fader (2008) reported 5 characteristics for achieving social continence are:
- to hold urine without leaking (which may be caused by an overactive bladder or urge incontinence)
- to keep the skin dry
- to be comfortable
- to be easy to put on
- to contain smell (which may be caused by urinary tract infection)
The table below demonstrates the top 5 characteristics that are required from a pad to provide effective continence management. These have been listed (in order of importance) for men and women with moderate to severe incontinence living in the community. You can clearly see that the most important element here is leakage.
MoliCare Premium Elastic with faster absorption core! The new MoliCare SkinGuard absorbent core technology offers an unparalleled level of protection and comfort for those with incontinence. The new absorption channel secures against leaks, providing an instant dry feeling and ensuring effective odour neutralisation. It is also pH balanced to maintain healthy skin and prevent skin irritation. Developed in consultation with caregivers, MoliCare Premium Elastic is ergonomic and easy to apply, making it an ideal all-in-one solution tailored for both users and carers.
Modern continence management is in the process of being been refined by a new continence product testing method known as ABL “Absorption Before Leakage”. ABL originated from EDANA, the European Disposables and Nonwovens Association. ABL is used to determine in “use absorption values of continence products for use on immobile people with moderate to severe incontinence. What this means is that we will no longer have to solely rely on the conventionally used ISO test method that involves soaking the entire continence product in fluid, which results in measuring only theoretically possible absorption capacities.
In contrast the, ABL test method simulates realistic test conditions, taking into consideration:
- Human body shape
- Pressure caused by body weight
- Positioning in bed
- Realistic voiding volumes released at the voiding point
These considerations determine realistic absorption capacities and will provide not only health professionals but consumers also, with a realistic assessment on continence product absorption capacities. This will facilitate easier selection of appropriate continence products to provide optimal social continence for those that are dependent on continence pads.
By Anthea Reus
Clinical Consultant Continence
Click here to download a PDF copy of this article.
This information is proudly brought to you by HARTMANN.

References
ABL (Absorption Before Leakage), source: standard test method WSP 354.1 (1)
Cottenden, A., Evidence based guidelines for product selection and use of continence products. Continence Foundation Australia Melbourne, 16-19 Nov 2011.
Fader, M., Cottenden, A., Getliffe, A., Gage, H., Clarke-O’Neill, S., Jamieson, N., Williams, P., Brooks, R., Malone_Lee, J. (Eds.) (2008). Absorbent products for urianry/faecal incontinence: a comparative evaluation of key product designs. Health Techonology Assessment 12(29), 1-208
ISO 11948-1:1996 Urine absorbing aids – part 1: Whole-product testing

